Regulating the immune system
A dissertation in the biochemical pharmacology research team at the University of Konstanz documents: The skin prevents inflammation by producing its own anti-inflammatory glucocorticoids
Too much of a good thing can be unhealthy, which is true for the human immune system as well: When immune responses go overboard, inflammation as well as damage to the respective tissue can be the result. Glucocorticoids are stress hormones that ensure that an immune response does not go out of control. They are mainly produced by the adrenal gland and circulated throughout the body via the bloodstream. They are also produced in epithelial tissues, which cover all the internal and external surfaces of the body. In his dissertation, Truong San Phan documents that the skin can produce its own glucocorticoids via the enzyme 11-beta-hydroxylase. The doctoral researcher in the biochemical pharmacology research team led by Professor Thomas Brunner at the University of Konstanz thus provides an important building block for future treatments of skin diseases such as atopic dermatitis and psoriasis. The results were published in the issue of 29 January 2021 of the journal Science Advances.
Epithelial tissues play an important role in the exchange of nutrients, gases and other signals between the human body and its surroundings. They also protect against invasion by potential pathogens. Damaged epithelial tissue is the basis for different inflammatory diseases in which glucocorticoids can be used very successfully as anti-inflammatory medicines. In the past, Thomas Brunner’s team was the first to document that glucocorticoids can be produced (synthesized) in the mucosal epithelia of the intestine and the respiratory epithelia of the lungs. In these locations, they ensure that the immune response does not go out of control and cause inflammatory diseases such as Crohn’s disease or asthma. Just like both internal organs, the skin also has contact with the external environment via the squamous epithelium and is also exposed to a great amount of bacteria, in addition to other harmful things.
Connection to the enzyme with an anti-inflammatory effect
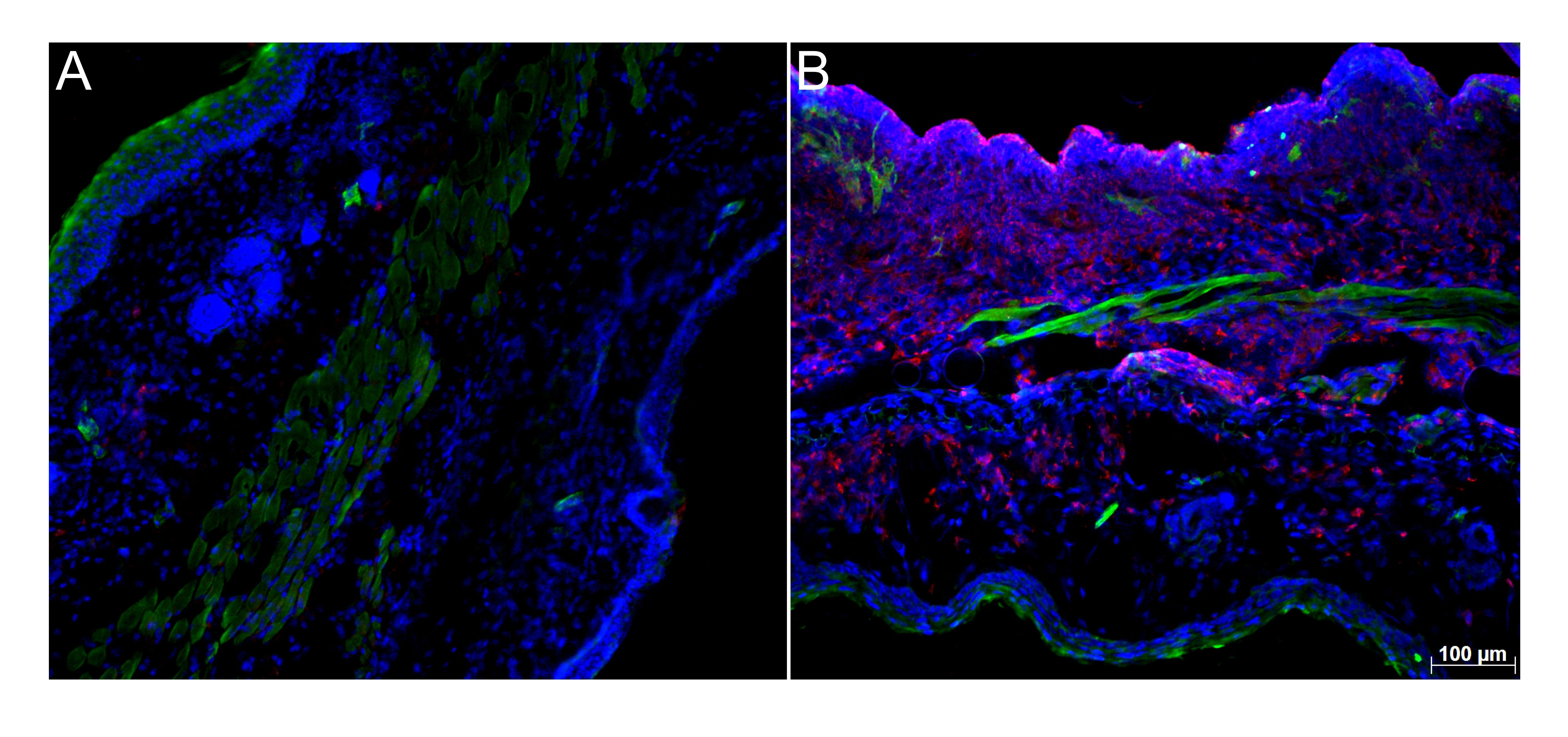
All three epithelia have their own complex immune system which is designed to protect the body from pathogens. There can, however, be too much of a good thing, and this is where the local synthesis of glucocorticoids plays an important role. Scientists have known that the enzyme 11-beta-hydroxylase plays a central role in the production of this stress hormone. What had not been documented until now was whether local glucocorticoid synthesis also plays a role in regulating local inflammatory processes. Truong San Phan was able to demonstrate that there is a connection between the presence of enzyme 11-beta-hydroxylase and skin disease. If there is an inflammation, then glucocorticoids are produced at the respective site, and the inflammation is reduced.
In patients with atopic dermatitis or psoriasis, however, the enzyme is practically non-existent. “The question of whether the skin disease arose because the enzyme was missing in the first place or whether the enzyme’s absence is caused by the skin disease can only be answered genetically”, Truong San Phan states. The doctoral researcher thus developed a mouse model in which the enzyme 11-beta-hydroxylase was specifically deactivated in keratinocytes, the most important skin cells. Afterwards the skin on the ears and back did, in fact, become inflamed, at the locations where enzyme production had been deactivated. “This means the skin locations were unable to produce bioactive immunoregulatory glucocorticoids”, explains Thomas Brunner. This demonstrates: “The skin itself produces the enzyme and the anti-inflammatory glucocorticoids”, says Truong San Phan.
What happens when the enzyme is missing
The mouse model with the deactivated gene clearly shows what happens when the enzyme is missing. The respective skin is populated by a large number of inflammatory cells. If the skin becomes damaged, then this opens the door for bacteria. The immune cells recognize the bacteria and either engulf them or travel to the lymph nodes where they cause a specific immune response. If these immunoregulatory glucocorticoids are no longer produced as a result of the missing enzyme, this can lead to uncontrolled inflammatory responses. As the study demonstrates, this is true for spontaneous inflammation in mice as well as for inflammatory responses like psoriasis.
“We think that this study can provide important new insights into what prevents inflammatory responses in the skin”, says Thomas Brunner. It is especially important to identify the factors that prompt the production of glucocorticoids. “When we understand what induces glucocorticoid synthesis in the skin, we can see whether these processes are active in patients with atopic dermatitis and psoriasis”, adds Thomas Brunner. If they are inactive, then it could be possible to use treatments that restore the body’s own regulation mechanism to induce endogenous glucocorticoid synthesis, with the goal of returning to the normal state.
Key facts:
- Original publication: Truong San Phan, Leonhard Schink, Jasmin Mann, Verena M. Merk, Pascale Zwicky, Sarah Mundt, Dagmar Simon, Dagmar Kulms, Susanne Abraham, Daniel F. Legler, Mario Noti, Thomas Brunner: Keratinocytes control skin immune homeostasis through de novosynthesized glucocorticoids, Science Advances, 29 January 2021. DOI: 10.1126/sciadv.abe0337
- Dissertation by Truong San Phan documents that enzyme 11-beta-hydroxylase enables the synthesis of anti-inflammatory glucocorticoids
- Completed in the biochemical pharmacology research group led by Professor Thomas Brunner
- Research project funded by the German Research Foundation (DFG) with approximately 260,000 euros
