| Universität Konstanz | Fachbereich Chemie |
||
Arbeitsgruppe Steiner |
|||
Photokinetik und Spinchemie |
|||
Photochemistry offers many possibilities to trigger specific molecular transformations by using the spectral, temporal or spatial controllability of light. In the past, our research was devoted to basic mechanistic studies of elementary photophysical and photochemical processes, such as electronic energy transfer, electron transfer, photoisomerizations or proton transfer. The control of chemical reactivity through spin and magnetic field effects on photochemically generated radical pairs has been a major research area [1]. Currently this line of research is still followed up in co-operations on novel applications of kinetic spin and magnetic field effects, for example in liquid nanodroplets encapsulated in transparent polymers [2].
Presently, the main focus of our research is directed to the study and improvement of the performance of photolabile protecting groups, especially with respect to their application for the light-directed synthesis of high-density DNA chips that are now widely used for genomic analysis. Meanwhile, high-density DNA-chips with up to several hundred thousand different spots on an area of 1 cm 2 can be synthesized. In principle, the complete human genome can be probed with such a chip. The required high spot densities can be achieved by photolithographic in-situ synthesis. In this method, the oligonucleotides are synthesized step by step from protected nucleoside phosphitamides using photolithography with masks or micromirror arrays as suitable means for achieving parallel spatial addressability. Such a technique requires photolabile protecting groups of high light sensitivity releasing their substrate (in this case a nucleotide) in as quantitative yield as possible. In a strategy to enhance the light sensitivity of photolabile protecting groups, we have exploited the principle of spectral sensitization [3]. This has led us to the synthesis of a novel generation of photolabile protecting groups, where the reacting group is covalently linked to an antenna molecule [4-7]. The principle is shown in Figure 1.
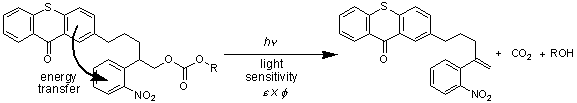
Figure 1. Example of a photolabile protecting group (the o-nitrophenylethyl moiety) with an intramolecularly linked antenna molecule (thioxanthone) [5]. The protected compound ROH is a nucleotide.
By using this principle, the light sensitivity at relevant wavelengths has been possible to improve by up to a factor of 25 with respect to the parent compound. While most of these investigations have been conducted in homogeneous solution, in a recent project we attempt to follow the kinetics of the photorelease of the protecting group by optical in-situ methods such as SPR (surface plasmon resonance) and RIFS (reflectometric interference spectroscopy) directly on a surface, i.e. under conditions that simulate the process of photolithographic DNA chip synthesis.
![]()
[1] U. E. Steiner and T. Ulrich, Chem. Rev.1989, 89, 51.,
[2] C. M. Elliott, U. E. Steiner, J. J. Kremer and K. A. Hötzer , Chem. Mater. , 2005, 17, 941
[3] a) S. Walbert, W. Pfleiderer and U. E. Steiner, Helv. Chim. Acta, 2001, 84, 1601; b) D. Wöll, S. Walbert, K.-P. Stengele, T. Albert, T. Richmond, J. Norton, M. Singer, R. Green, W. Pfleiderer and U. E. Steiner, Helv. Chim. Acta , 2004, 87, 28.
[4] J. Smirnova, D. Wöll, W. Pfleiderer and U. E. Steiner, Helv. Chim. Acta, 2005, 88, 891.
[5] D.Wöll, J. Smirnova, W. Pfleiderer and U.E. Steiner, Angew. Chemie. Int. Ed., 2006, 45 , 2975-2978.
[6] D.Wöll, M. Galetskaya, J. Smirnova, W. Pfleiderer, W. Heinz, P. Gilch and U.E. Steiner, J. Am. Chem. Soc. 2007, 129 , 12148-12158.
[7] D.Wöll, J. Smirnova, M. Galetskaya, T. Prykota, K.P. Stengele, W. Pfleiderer and U.E. Steiner, J. Chem. Eur. J. 2009, 14, 6490-6497.