Novel rapid test method for Covid-19 developed on the basis of innovative DNA polymerases
A research cooperation at the University of Konstanz led by Professor Christof Hauck (Department of Biology) with participation of the Hospital of Konstanz, a Konstanz diagnostics laboratory and the Konstanz-based company myPOLS Biotec, a spin-off of the working group Organic Chemistry / Cellular Chemistry at the University of Konstanz, has developed a novel rapid test method for Covid-19. This test makes it possible to obtain results in half the time compared to the conventional polymerase chain reaction (PCR).
Early identification of patients infected with the novel coronavirus SARS-CoV-2 is a crucial pre-requisite for the global management of the current pandemic. Here, the polymerase chain reaction (PCR) is the standard test procedure used by diagnosticlaboratories in Germany for detection of acute infection. This method is based on an enzyme, a so-called DNA polymerase, replicating the genetic material of the pathogen, thereby making it visible. Such a DNA-based PCR test is very robust and extremely sensitive, but in the case of an RNA virus such as SARS-CoV-2 it has one decisive disadvantage: Two preliminary steps are required, in which the RNA of the virus is first purified and then enzymatically converted from RNA to DNA. Only then can the actual amplification by DNA polymerase take place. Due to global demand, bottlenecks in reagents and consumables for the automated purification of RNA keep occurring, and manual isolation of RNA from patient samples is error-prone and time-consuming; hence there is great interest in alternative approaches to detectingthe RNA of the coronavirus.
Scientists at the University of Konstanz have now presented an innovative method to obviate the time-consuming and costly purification of the viral RNA. This new procedure is made possible by the use of an optimized enzyme that plays a dual role: The enzyme can work both as an RNA-dependent polymerase that converts the SARS-CoV-2 genome from RNA to DNA, and as a DNA-dependent polymerase that immediately starts replication of the DNA. Since this enzyme is also particularly stable, both steps can be carried out at high temperatures, at full steam, so to speak.
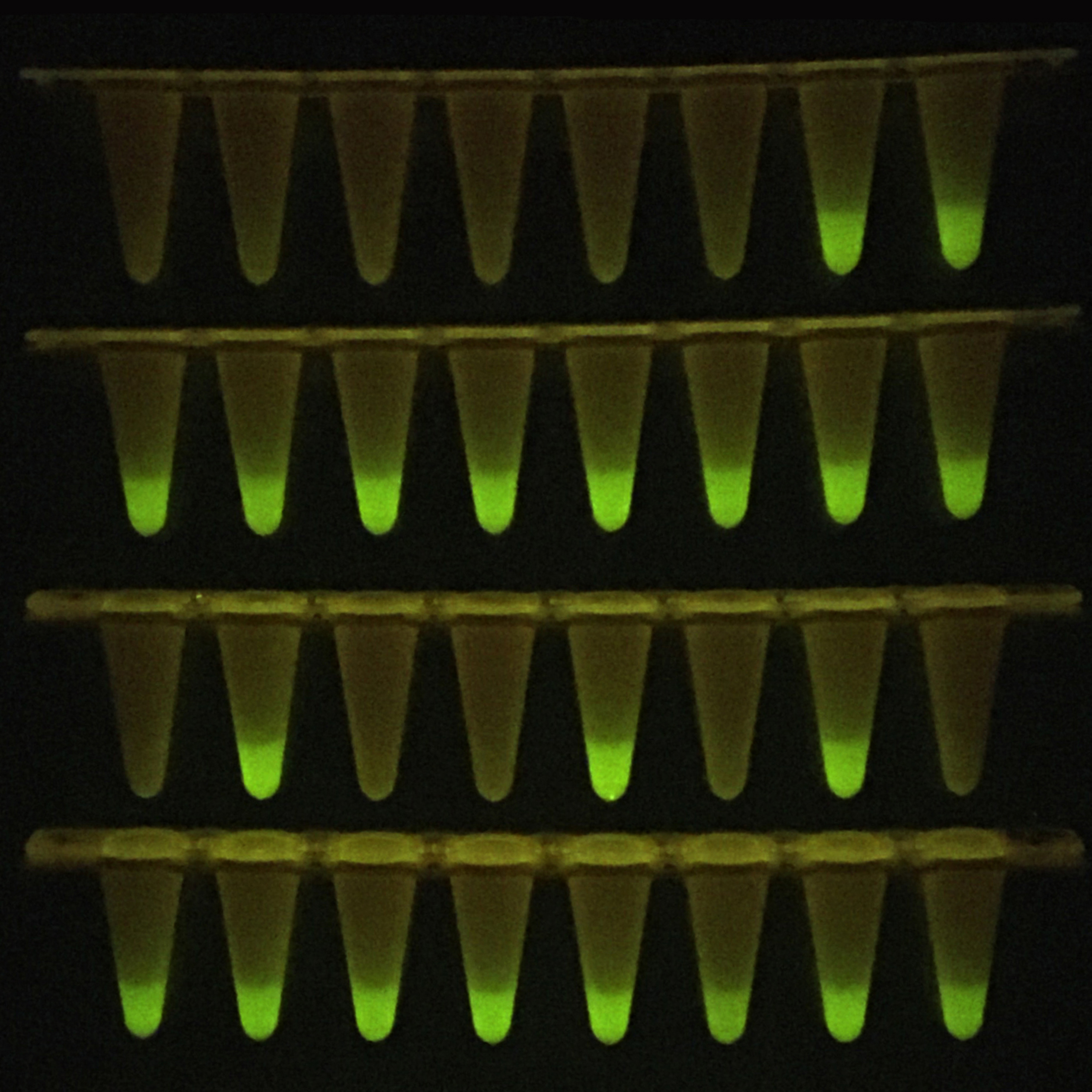
It has been shown that this enables SARS-CoV-2 detection starting directly from patient material, thus obviating the resource-and time-consuming RNA purification step. Whether an infection is present or not is therefore discerned much earlier with this test: After only two hours. The sensitivity of the test is similarly high as that of conventional PCR.
Apart from saving time, the new SARS-CoV-2 detection method offers the major advantage of straightforward implementation: Dispensing with the time-consuming RNA isolation eliminates several steps of sample handling, so the risk of carry-over from individual samples is minimized, especially when working at high sample throughput. Simplification can be achieved with this new approach also due to the fact that conventional PCR equipment can be used, and the test result can be read out not only in an exclusive diagnostic device but also by means of an unpretentious portable LED lamp. Coupled with the possibility of avoiding the cost-intensive isolation of RNA, this new method is therefore particularly suitable for those regions of the world where Covid-19 is currently spreading rapidly but diagnostic standard procedures far exceed economic resources.
Key facts:
- The results were published on medRxiv.org as a so-called preprint version. The paper reports on new medical research results that still need to be evaluated and therefore cannot serve as a guideline for clinical practice yet.
- Preprint publication: Johannes W. P. Kuiper, Timo Baade, Marcel Kremer, Ramon Kranaster, Linda Irmisch, Marcus Schuchmann, Johannes Zander, Andreas Marx, Christof R. Hauck, Detection of SARS-CoV-2 from raw patient samples by coupled high temperature reverse transcription and amplification, medRxiv.org, 19.05.2020. DOI: https://doi.org/10.1101/2020.05.19.20103150
- A research cooperation led by Professor Christof Hauck (Department of Biology) at the University of Konstanz has developed a novel rapid test method for Covid-19 that is based on DNA polymerases.
- This new method does not require RNA purification and is therefore not only faster but also considerably cheaper than conventional PCR is, costing less than € 5 per test.The sensitivity of the method is similarly high as that of conventional PCR.
- The partner institutions include the Hospital of Konstanz (collection of patient samples) and the Konstanz-based diagnostics laboratory Labor Dr Brunner. The latter performed the comparator measurements with the established CE-certified routine method (PCR).Also involved is myPOLS Biotec, a spin-off from the working group Organic Chemistry / Cellular Chemistry at the University of Konstanz led by Professor Andreas Marx and Dr Ramon Kranaster (now managing director of myPOLS Biotec). The new test method is based on a DNA polymerase developed by myPOLS.
- Timo Baade (from the research team of Professor Hauck) confirms the financial support of the project by the Konstanz Research School Chemical Biology (KoRS-CB) at the University of Konstanz. This concerns third-party funding from the Excellence Initiative as well as budget funds. Professor Andreas Marx confirms the –non-financial –support of the project by the German Research Foundation (DFG) within the framework of SPP 1784.
Read the article in our online magazin campus.kn (in German).
