ERC Consolidator Grant for Konstanz chemist
The European Research Council awards a Consolidator Grant to Professor Tanja Gaich from the University of Konstanz for a project on the synthesis of highly complex molecules from the taxane family through structural deconvolution. Funding is provided for the further development and testing of a new strategy for the synthesis of natural substances that could not previously be produced in the laboratory.
For her research project entitled “Cooperative Synthesis by Molecular Deconvolution” (CoSyMoDe), Professor Tanja Gaich from the Department of Chemistry at the University of Konstanz receives a five-year Consolidator Grant from the European Research Council (ERC) worth up to two million euros. She will use the funding to further develop a new strategy for the synthesis of highly complex natural product molecules, which will simplify conventional processes and make it possible to produce natural products in the laboratory that previously could not be synthesized.
This is the second ERC Grant Tanja Gaich could successfully acquire. In 2015 she was already awarded an ERC Starting Grant for an unrelated research project and thus is now the first scientist at the University of Konstanz to have acquired two ERC research grants.
Why natural product synthesis matters
Tanja Gaich leads the Organical Chemistry research team at the University of Konstanz. She studies the synthesis of complex polycyclic natural products, focusing on the development of efficient synthesis methods to produce them in the laboratory.
Natural products are secondary metabolites that do not primarily serve the life support of an organism. These include, for example, the chemical substances that a fungus releases to fend off potential competitors, giving it an evolutionary advantage. “Drugs such as penicillin (antibiotic) and the vast majority of all pharmaceutical agents we know are based on natural substances,” explains Tanja Gaich. Usually, only very small quantities of these substances, which are highly effective and very interesting for humans, can be found in nature. Consequently, the synthetic production is absolutely essential for potential pharmaceutical applications.
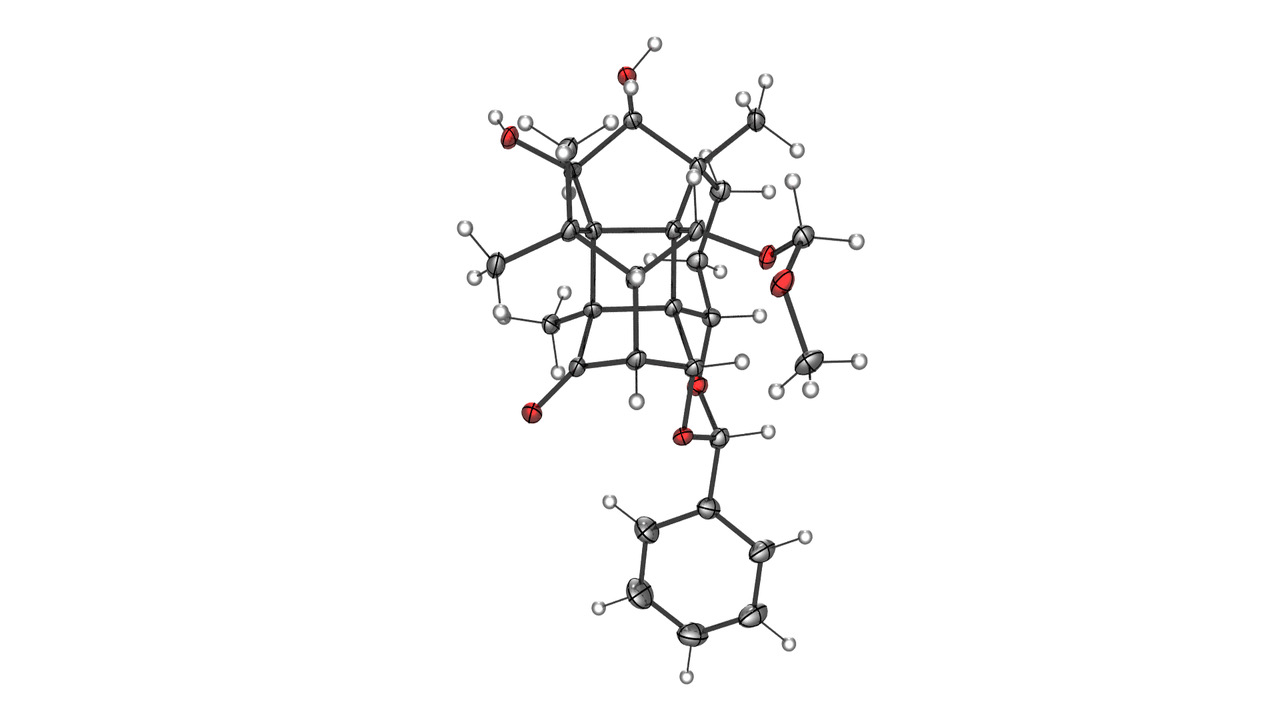
Synthesis of highly complex taxanes
This also applies to the family of taxanes, a very large group of natural substance molecules that occur in hundreds of different forms in yew trees. Taxol, a natural substance based on it, has been used in cancer treatment for over 30 years and is one of the best-selling tumour therapeutics. While the problem of lacking raw material in this case could be solved years ago, the subgroup of complex taxanes, from which science also expects a high pharmaceutical benefit, remains largely unexplored.
“The reason is that these taxanes are very complex, only occur in incredibly small amounts and could not be synthesized at all until now”, Tanja Gaich says. At the beginning of 2020, her research team succeeded in synthesizing canataxpropellane, one of the most complex natural substances that has ever been replicated in a laboratory. Building on this success, Gaich intends to use the funding from the ERC Consolidator Grant to synthesize the other molecules of this group, too.
Structural deconvolution
To reach this goal, she developed a new strategy which has decisive advantages over conventional synthesis methods. “In contrast to the prevailing methodology, we take the most complex molecule from the family, in this specific case the canataxpropellane, which we have already successfully synthesized, and convert it into the other molecules by means of structural deconvolution.” In this process the already synthesized structure is gradually and systematically simplified, the particular challenge being the selective and customized transformation of the molecule. Up to now, the most simple component of a complex molecule has been used as the basis for synthesis. The decisive advantage of cooperative synthesis by structural deconvolution is that it is not necessary to provide a separate synthesis pathway for each complex molecule. Instead, the one-time synthesis of the most complex building block makes it possible to derive all other, simpler relatives from it.
“The process is much more manageable in terms of effort, allowing us to develop new chemical reactions and then to correlate the structure and activity of the natural substances produced in this way in order to test their effectiveness,” says Gaich. If this results in a pattern, a rational drug design is possible in principle: “Based on theory we can say quite reliably which changes in the molecule must have which consequences due to the structure-activity relationship. This is particularly interesting because this way molecules can be produced that no longer have anything to do with the natural substance but can be relevant for pharmaceutical applications. However, we still have a long way to go.”
Optimizing the effects of natural substances in the body
Irrespective of the ERC Consolidator Grant, Tanja Gaich is also involved in a joint research project with Professor Christine Peter (Department of Chemistry) and Professor Thomas Mayer (Department of Biology) at the University of Konstanz, which focuses on the structure-activity relationship of molecules. This Blue-Sky project is financed with funds from the Excellence Strategy at the University of Konstanz. The scientists combine their expertise in synthesis, molecular modelling and cell biology to study natural products in relation to their biological target. “In simple terms, we look at where and how the natural substance acts in the cell. This way we gain insights into what we need to optimize so that the natural substance and biological structure become an even closer fit.”
Facts:
- ERC Consolidator Grant 2020 for Tanja Gaich, professor of organical chemistry at the University of Konstanz.
- Funding for the project “Cooperative Synthesis by Molecular Deconvolution” (CoSyMoDe) to further develop a new, simplified method for natural product synthesis.
- In contrast to the prevailing methodology, which uses a separate synthesis pathway for each complex molecule, CoSyMoDe starts with the most complex molecule of a group. By systematically simplifying its structure, all other related molecules can be derived from it through certain chemical reactions.
- Testing using the example of highly complex taxane molecules, which could not be produced in the laboratory up to now, but might be considered for pharmaceutical development.
- Funding sum: up to two million euros.
- Funding period: five years.
