Taking the sting out of a stomach germ
Konstanz cell biologists carry out successful research on the pathogen causing stomach cancer.
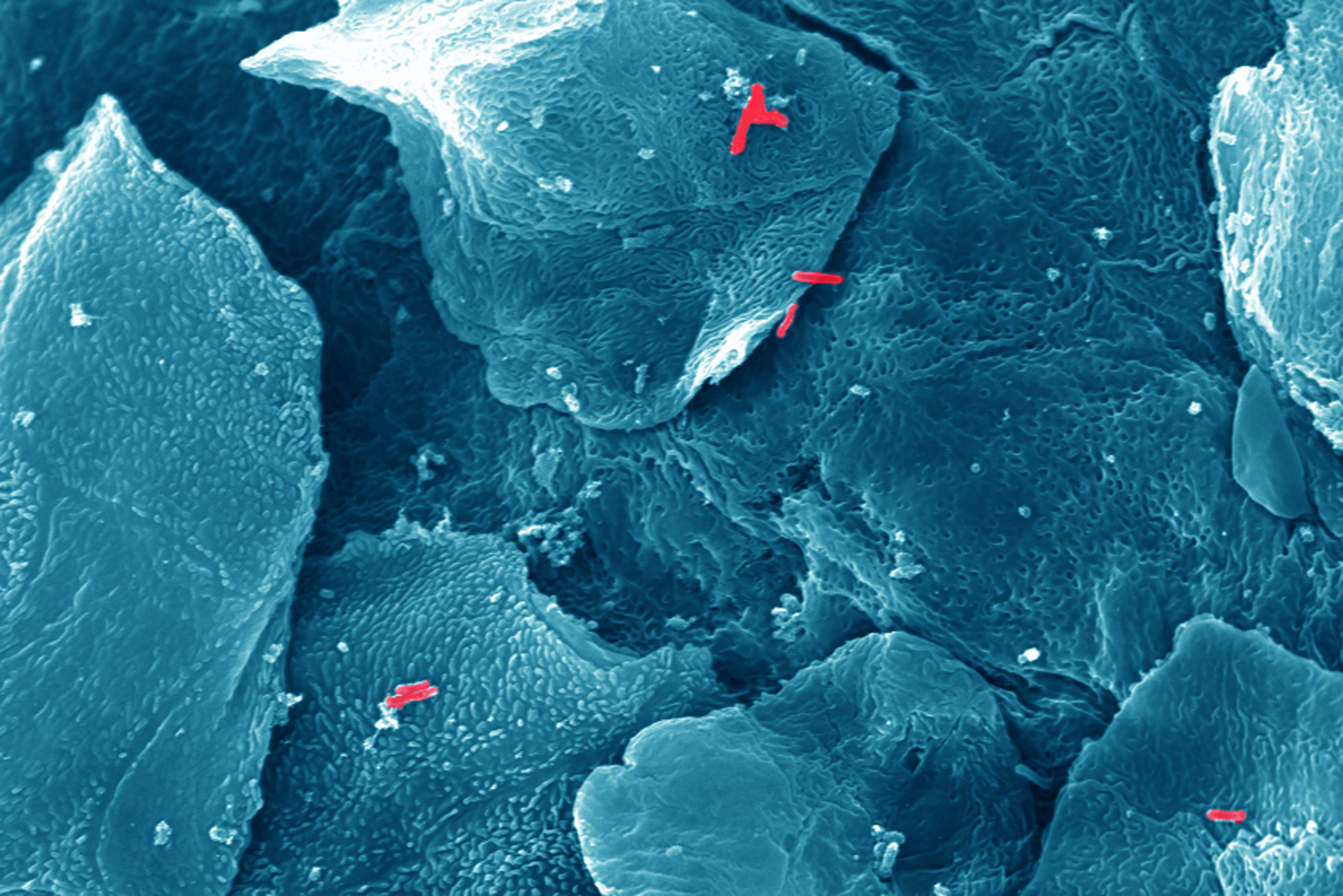
Anyone who had a stomach ulcer knows how painful an encounter with Helicobacter pylori can turn – the bacterial pathogen responsible for infection and inflammation of the human stomach. This microbe, inhabiting the stomachs of around four billion people worldwide, has been identified as a cause of gastric cancer. Accordingly, Helicobacter pylori is the only bacterium classified as carcinogenic by the World Health Organization. New insights gained by Professor Christof Hauck's team of cell biologists at the University of Konstanz together with Prof. Rainer Haas’ team at Ludwig-Maximilians University (LMU) and Markus Gerhard’s team at the Technical University of Munich (TUM) might now provide a new avenue to disarm this pathogen. The researchers identified the molecular point of contact between Helicobacter and the gastric mucosa. Exactly pinpointing the details of this tight interaction is pre-requisite to the development of inhibitors, which disrupt the connection and thus prevent the development of disease. The results were published in two publications in the renowned scientific journal "Nature Microbiology" on 17 October 2016.
Not all Helicobacter variants are evil. Predominantly those strains carrying a special weapon, the CagA protein, are associated with gastric cancer. In the course of an infection, the pathogen actively delivers CagA into the gastric cells of its human host. It is believed that CagA triggers alterations in normal mucosal cells, which finally result in tumour development.
To transfer the CagA protein into cells, the bacteria must be in direct contact with the host tissue. "To do so, the bacteria use a 'molecular boat hook', an adhesin, to attach to receptors on the surface of mucosal cells", explains Christof Hauck. So far, neither the adhesin used by Helicobacter pylori, nor the matching human receptor had been known. This is where the researchers from Konstanz and Munich started: the biologists first identified the receptor on the cell surface of the gastric cells that acts as docking site. The fact th at the research team around Professor Hauck had described these receptors as an important point of attachment for pathogenic bacteria in a recently published publication facilitated the search. Knowing the human receptor then enabled the researchers to find the adhesin of Helicobacter, the protein HopQ, and to resolve its atomic structure.
Exactly determining this point of contact between pathogen and tissue is an important pre-requisite for developing active substances that prevent the bacterium from binding to human cells. The researchers show that deletion of HopQ from the bacteria, or alternatively blockade of the receptor on human cells, disrupts the transfer of CagA into isolated cells. "If we are able to intercept the adhesion of Helicobacter and thus prevent the delivery of CagA in the tissue, this might inhibit the process of tumour development", says Professor Hauck about the medical potential of the new findings.
Facts:
The research was conducted in collaboration with the teams of Professor Markus Gerhard, Technical University of Munich (TUM), as well as Professor Rainer Haas, Ludwig-Maximilians University Munich (LMU).
The research project was funded by the German Research Foundation (DFG) in the context of the project "The role of CEACAM-recognition for mucosal colonization by human-specific bacterial pathogens".
Original publication:
Königer V., Holsten L., Harrison U., Busch B., Loell E., Zhao Q., Bonsor D.A., Roth A., Kengmo-Tchoupa A., Smith S.I., Mueller S., Sundberg E.J., Zimmermann W., Fischer W., Hauck C.R. and Haas R. (2016) Helicobacter pylori exploits human CEACAMs via HopQ for adherence and translocation of CagA. Nature Microbiology
DOI: 10.1038/nmicrobiol.2016.188
Javaheri A., Kruse T., Moonens K., Mejías-Luque R., Debraekeleer A., Asche I., Tegtmeyer N., Kalali B., Bach N.C., Sieber S.A., Hill D.J., Königer V., Hauck C.R., Moskalenko R., Haas R., Busch D.H., Klaile E., Slevogt H., Schmidt A., Backert S., Remaut H., Singer B.B. and Gerhard M. (2016) Helicobacter pylori adhesin HopQ engages in a virulence-enhancing interaction with human CEACAMs. Nature Microbiology
DOI: 10.1038/nmicrobiol.2016.189
Prior work was published in the scientific journal PLoS Pathogens in 2016:
Muenzner P., Kengmo Tchoupa A., Klauser B., Brunner T., Putze J., Dobrindt U. and Hauck C.R. (2016) Uropathogenic E. coli exploit CEA to promote colonization of the urogenital tract mucosa. PLoS Pathog.
DOI: 10.1371/journal.ppat.1005608
