The role of fungi during establishment and decay of reed (Phragmites australis)
 |
Fungi play important and diverse roles in terrestrial ecosystems. They are the main decomposers of organic material, act as pathogens of plants and animals, or may provide benefits to them. Despite their importance and after well over a century of mycological research, still little is known of the true diversity of fungal populations in nature. A number of 1.5 million fungal species has been given as a conservative estimate of fungal diversity on earth. Of these, only 5-10% have been described so far.
We have extensively studied the mycoflora of an important wetland grass, common reed (Phragmites australis) at two locations on Lake Constance, applying both traditional, culture-based and molecular methods to elucidate different aspects of fungal diversity in reed stands of Lake Constance. Identification and phylogenetic classification of the isolates are carried out using morphological features along with ITS-sequence data. The abundance of some of the most important fungi is being investigated using nested PCR assays with specific primers. Light and electron microscopy methods are applied to study colonisation processes and interactions between different reed colonisers. The functional significance of fungal or oomycete strains for the performance and vitality of reed plants and their interactions is tested in micro- and mesocosm experiments and in infection assays.
Using PCR-based methods, we found an unexpectedly high diversity of fungi associated with reed, as it has probably not been shown for any other plant species. Many of the approx. 400 different RFLP restriction patterns we identified possibly represent new, previously unrecognised fungal taxa (Figure 1). Some of the dominating, previously undescribed fungi could be isolated via a combination of culture-based and molecular techniques.
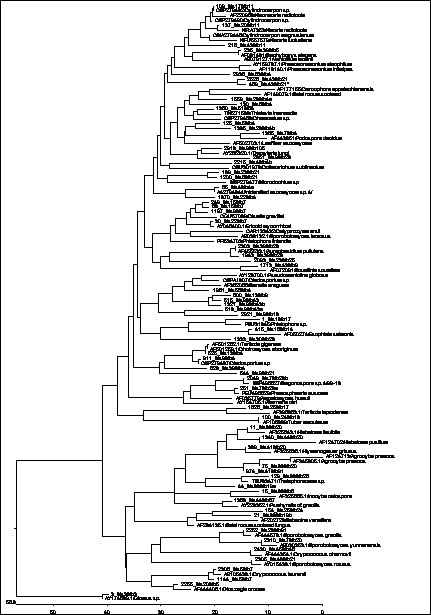 |
Fig. 1: ITS phylogeny of sequenced fungi associated with common reed and closest relatives according to BLAST searches. Separate branches comprising reed taxa indicate the occurrence of several new taxa at different taxonomic levels. |
Plants infected with members of the genus Stagonospora showed significantly better growth in microcosm experiments. These fungi, well-known as endophytes of agronomic grasses, possibly take over the role of mycorrhizal fungi on flooded sites (Figure 2).
 |
Fig. 2: Effect of Stagonospora spp. on growth of P. australis in microcosms. Left: control, no inoculum. Right: inoculation with Stagonospora sp. |
High-resolution cryo-scanning electron microscopy revealed that Cladosporium isolates from reed are very diverse. Four different species were found to sympatrically colonise reed plants (Figure 3).
 |
Fig. 3: Low-temperature scanning electron microscopy of conidia from Cladosporium isolates from reed. A: C. oxysporum; B: C. oxysporum; C: C. herbarum; D: Cladosporium sp |
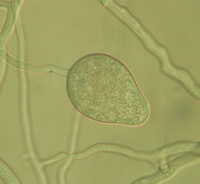
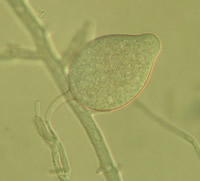
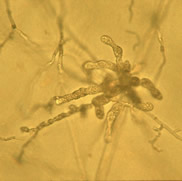
Lake Constance reed stands were also shown to harbour a diverse flora of oomycetes of the important plant pathogen genera Pythium and Phytophthora. Most taxa represent new, undescribed species of both genera (Figure 4).
 |
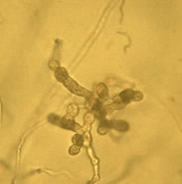 |
Fig. 4: Sporangia of Phytophthora sp. (left) and Pythium sp. (right) isolated from Lake Constance reed stands |
 |
 |
Some species are likely to play a role during the establishment of new reed stands, as they were shown to be highly aggressive towards reed seedlings under controlled conditions (Figure 5). Currently, the diversity of oomycetes associated with reed is being investigated using PCR-based methods.
 |
Fig. 5: Performance of reed seedlings growing in substrate infected with Pythium sp. (bottom), as compared to control plants (top). |
Fungi and omycetes may also be involved in reed decline phenomena (“reed die-back”) that have repeatedly been reported for Lake Constance and other European freshwater lakes (Figures 6 - 8).
 |
Fig. 6: Die-back of reed. Picture taken in September 2000. |
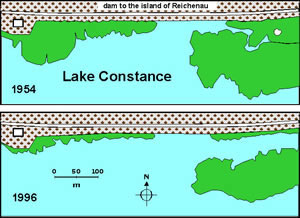 |
Fig. 7: Decline of reed along the dam to the island of Reichenau from 1954 to 1996 |
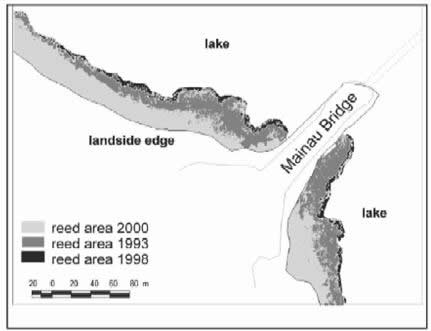 |
Fig. 8: Development of reed die-back in Lake Constance-Obersee 1993-2000 (from Ostendorp, W.; Dienst, M.; Schmieder, K., 2003: Hydrobiologia 506–509, 687–695). |
The project is funded by the Deutsche Forschungsgemeinschaft as part of the SFB 454 (Littoral Zone of Lake Constance)
Contact: Jan Nechwatal, Anna Wielgoss
- Molecular characterisation of fungal diversity in reed
- Molecular characterisation of oomycete diversity in reed
- Isolation of specific reed-associated fungi detected and identified by molecular methods
- Identification, characterisation and pathogenicity of new Pythium spp. pathogenic to reed
- Dynamics of Pythium-mediated dieback of reed
- Identification, characterisation and pathogenicity of new Phytophthora spp. from the Lake Constance litoral
Publications:
· Nechwatal, J., & Mendgen, K. 2009. Evidence for the occurrence of natural hybridization in reed-associated Pythium species. Plant Pathology, 58, 261-270.
· Hirsch, P. E., Nechwatal, J. & Fischer, P. 2008. A previously undescribed set of Saprolegnia spp. in the invasive spiny chee crayfish (Orconectes limosus, Rafinesque). Fundamental and Applied Limnology Archiv für Hydrobiologie, 172, 161-165.
· Jung, T. & Nechwatal, J. 2008. Phytophthora gallica sp. nov., a new species from rhizosphere soil of declining oak and reed stands in France and Germany. Mycol. Res., 112, 1195-1205.
· Nechwatal, J., Wielgoss, A. & Mendgen, K. 2008. Diversity, host, and habitat specificity of oomycete communities in declining reed stands (Phragmites australis) of a large freshwater lake. Mycol. Res., 112, 689-696.
· Nechwatal, J., Wielgoss, A., and Mendgen, K., 2008: Flooding events and rising water temperatures increase the significance of the reed pathogen Pythium phragmitis as a contributing factor in the decline of Phragmites australis. Hydrobiologia, 613, 109-115. .
· Nechwatal, J., and Mendgen, K., 2006: Pythium litorale sp. nov., a new species from the littoral of Lake Constance, Germany. FEMS Microbiology Letters 255, 96-101.
· Nechwatal, J., and Mendgen, K., 2006: Widespread detection of Phytophthora taxon salixsoil in the littoral zone of Lake Constance, Germany. European Journal of Plant Pathology, 114, 261-264.
· Gao, K., and Mendgen, K. 2006: Seed transmitted beneficial endophytic Stagonospora sp. can penetrate the walls of the root epidermis, but does not proliferate in the cortex of Phragmites australis. Canadian Journal of Botany, 84, 981-988..
· Nechwatal, J., and Mendgen, K., 2006: Pythium litorale sp. nov., a new species from the littoral of Lake Constance, Germany. FEMS Microbiology Letters, 255, 96-101.
· Neubert, K., Mendgen, K., Brinkmann, H., and Wirsel, S.G.R. 2006: Only few fungal species dominate highly diverse mycofloras associated with the common reed. Applied and Environmental Microbiology, 72, 1118-1128.
· Nechwatal, J.; Wielgoss, A.; Mendgen, K., 2005: Pythium phragmitis sp. nov., a new species close to P. arrhenomanes as a pathogen of common reed (Phragmites australis) Mycol. Res. 109, 1337-1346.
· Damm, U.; Brune, A.; Mendgen, K.W., 2003: In vivo observation of conidial germination at the oxic-anoxic interface and infection of submerged reed roots by Microdochium bolleyi. FEMS Microbiol. Ecol. 45, 293-299.
· Ernst, M.; Mendgen, K.W.; Wirsel, S.G.R., 2003: Endophytic fungal mutualists: seed-borne Stagonospora spp. enhance reed biomass production in axenic mircocosms. Mol. Plant Microbe Interact. 16, 580-587.
· Wirsel, S.G.R., 2003: Homogenous stands of a wetland grass harbour diverse consortia of arbuscular mycorrhizal fungi. FEMS Microbiol. Ecol. 48, 129-138.
· Wirsel, S.G.R.; Leibinger, W.; Ernst, M.; Mendgen, K., 2001: Genetic diversity of fungi closely associated with common reed. New Phytol. 149, 589-598.
· Wirsel, S.G.R.; Runge-Froböse, C.; Ahren, D.G.; Kemen, E.; Oliver, R.P.; Mendgen, K.W., 2002: Four or more species of Cladosporium sympatrically colonize Phragmites australis. Fung. Genet. Biol. 25, 99-113.
 |